Retinotopic Mapping: Exploring the Functional Organization of the Mouse Visual Cortex
The visual system of mammals is organized in a way that each section of the visual field is processed by a corresponding region of the brain. This is known as a visuotopic or retinotopic (referring to the retina) organization of the visual system. The retinotopic mapping of the visual cortex was previously described in several mammalian species – including primates, carnivores and rodents – using different anatomical or functional approaches. Each approach comes with advantages and drawbacks.
Generally speaking, anatomical methods for retinotopic mapping (e.g. histological assessment of tracers) provide a more detailed visualization of the topographic organization of neurons (at the cellular level) with the caveat that this procedure is necessarily post-mortem. This implies that the retinotopic organization of the visual cortex of a particular individual is known after all tests/interventions are made which increases the rate of errors. In contrast, functional methods can be used during an experiment session to first obtain the retinotopic map and then use this information to target other interventions, for example. Among the functional methods available, optical imaging is one of the most used in animal research.
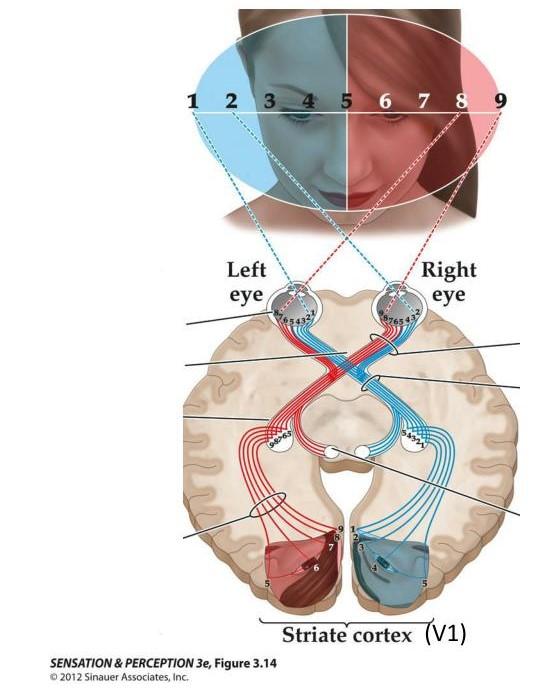
The Technique for Retinotopic Mapping
Optical imaging has been a key technique in neuroscience research for nearly 40 years. In brief, this method consists of illuminating the brain and detecting small fluctuations in light intensity using a highly sensitive camera. These fluctuations can arise from endogenous (intrinsic) signals or exogenous signals, such as calcium indicators and voltage-sensitive dyes, under different illumination wavelengths. The source of the signal and the wavelength used to illuminate the brain determine the specific aspects of cerebral physiology being observed. These fluctuations can provide valuable insights into different domains of brain function, including neuronal activity, metabolic processes, and vascular dynamics. These signals are recorded at a mesoscopic scale, allowing for the imaging of relatively large brain surfaces (several millimeters) simultaneously. This scale provides a significant advantage in recording the neurovascular physiology of small animals. Taken together, these features make optical imaging a simple and yet versatile technique to assess brain function in animal research. Our OiS200 LightTrack Optical Imaging System is well-suited in this context, offering multi-wavelength recordings at a mesoscopic scale. This makes it a suitable option for comprehensive assessments of brain function in various neuroscience research applications.
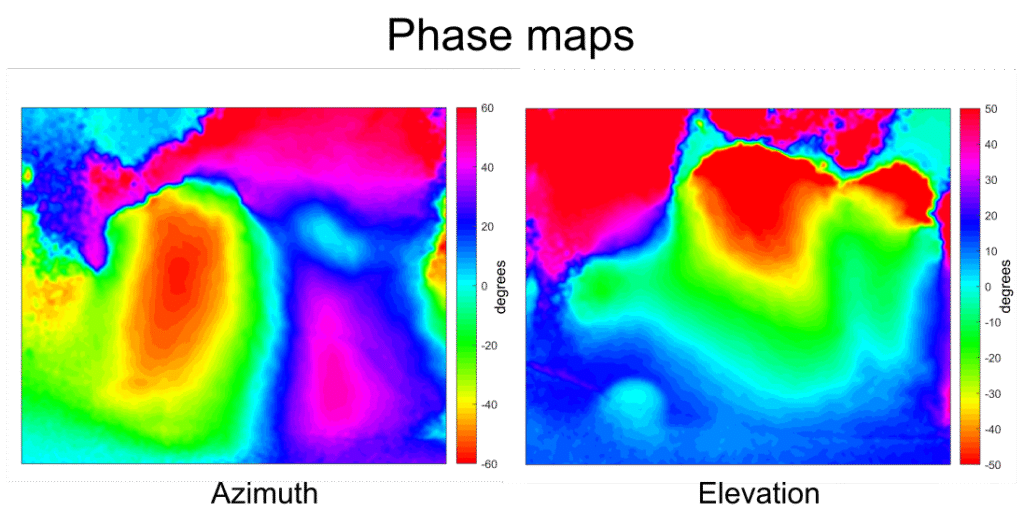
In the context of the visual system, Kalatsky and Stryker (2003) proposed a retinotopic mapping procedure using this approach. A bar drifts periodically across a screen in the animal’s visual field in four cardinal directions. By performing Fourier analysis of the cortical responses, the phase component (phase maps) of the signal at the bar’s temporal frequency for each direction is extracted. These phase maps are then combined to create retinotopic maps for the horizontal (azimuth) and vertical (elevation) axes, where each color corresponds to the position of the bar in the visual field.
The most common method for retinotopic mapping used today was popularized in the optical imaging field by Kalatsky and Stryker (2003). In their paper, they described a novel approach for the processing of optical imaging signals. Traditionally, brain responses were calculated from the presentation of transient (episodic) sensory stimuli, which were then averaged and normalized to a control condition. In contrast, their technique involves presenting a periodic stimulus and performing a Fourier analysis of the brain responses at the stimulus frequency. This alternative approach significantly reduces the time required to obtain response maps compared to the conventional method.
Retinotopic Mapping in Mice
With a large portfolio of transgenic animals available, the mouse has become an invaluable model in the field of neurosciences. In addition, its small brain size and smooth cortex allow the visualization of virtually all the neocortex at a mesoscopic scale at once, which makes the optical imaging of the mouse cortex a very useful technique to investigate the brain’s function and structure.
Functional Organization of the Visual Cortex
The analysis of retinotopic maps provides important insights into the functional organization of the visual cortex. For example, studies have assessed different features of retinotopic maps such as map size, shape, and phase distribution to investigate the impact of specific proteins on visual processing using mouse knock-out models (Souza et al. 2018; Groleau et al. 2014; Greifzu et al. 2016), while others have used the technique to characterize the visual aptitudes of different mouse strains (Yeritsyan et al. 2012).
Identification of Visual Areas
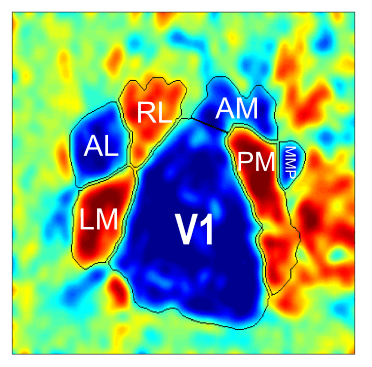
Another usage of retinotopic maps in mice involves the identification of individual visual cortical areas. Recent studies have used retinotopic mapping to identify more than 10 visual cortical areas (Garrett et al. 2014; Zhuang et al. 2017). The functional segmentation of individual areas is possible due to a particular feature of the topographical organization of the visual cortex, where neighboring distinct areas show a reversing (mirroring) of the retinotopic gradients. One can use this feature to create a map (visual sign map) showing the regions of reversing gradients as the borders of visual areas.
The identification of visual areas by retinotopic mapping allows researchers to draw a more precise profile of the properties of the visual cortex by creating a unique map for each mouse. These individualized maps can be used to assess the neuronal function across the areas using mesoscopic optical imaging or other techniques such as electrophysiology (Nsiangani et al. 2022) or two-photon microscopy (Marshel et al. 2011) as well as a guide to target a specific area for injections of viruses or neuronal tracing agents (Mocanu and Shmuel 2021).
Retinotopic Mapping: Other Applications and Future Directions
Retinotopic mapping in mice, when combined with other optical imaging techniques such as calcium imaging, offers a powerful tool for assessing visual function and structure in the context of longitudinal studies in awake mice. For instance, researchers can evaluate how the retinotopic maps of a given mouse change over time following trauma, injury, or pharmacological disruption of brain function. This allows for the observation of dynamic processes and recovery patterns in a more naturalistic setting (e.g., without the confounding effects of anesthesia). Additionally, longitudinal studies can help us understand the progression of neurodegenerative diseases, the effects of long-term drug treatments, and the impact of genetic modifications on visual processing.
By enabling repeated measurements in the same animal, these techniques reduce variability and improve the reliability of the data. In this context, the optical imaging technique is an invaluable tool for answering complex questions about brain function and plasticity that are difficult to address with traditional approaches.
In addition, retinotopic mapping has proven to be an invaluable method in the study of the visual cortex, particularly in mice. This technique not only allows for the detailed functional analysis of the visual cortex but also provides a faster and more precise way to identify distinct visual areas. As advancements in optical imaging technologies and genetic tools continue, the applications of retinotopic mapping are likely to expand, offering even deeper insights into the complexities of brain function.
Products Used
References
Garrett, Marina E., Ian Nauhaus, James H. Marshel, and Edward M. Callaway. 2014. “Topography and Areal Organization of Mouse Visual Cortex.” The Journal of Neuroscience 34 (37): 12587–600. https://doi.org/10.1523/JNEUROSCI.1124-14.2014.
Greifzu, Franziska, Daniel Parthier, Bianka Goetze, Oliver M. Schlüter, and Siegrid Löwel. 2016. “Ocular Dominance Plasticity after Stroke Was Preserved in PSD-95 Knockout Mice.” PLOS ONE 11 (3): e0149771. https://doi.org/10.1371/journal.pone.0149771.
Groleau, Marianne, Hoang Nam Nguyen, Matthieu P. Vanni, Frédéric Huppé-Gourgues, Christian Casanova, and Elvire Vaucher. 2014. “Impaired Functional Organization in the Visual Cortex of Muscarinic Receptor Knock-out Mice.” NeuroImage 98 (September):233–42. https://doi.org/10.1016/j.neuroimage.2014.05.016.
Kalatsky, Valery A., and Michael P. Stryker. 2003. “New Paradigm for Optical Imaging.” Neuron 38 (4): 529–45. https://doi.org/10.1016/S0896-6273(03)00286-1.
Marshel, James H., Marina E. Garrett, Ian Nauhaus, and Edward M. Callaway. 2011. “Functional Specialization of Seven Mouse Visual Cortical Areas.” Neuron 72 (6): 1040–54. https://doi.org/10.1016/j.neuron.2011.12.004.
Mocanu, Victor M., and Amir Shmuel. 2021. “Optical Imaging-Based Guidance of Viral Microinjections and Insertion of a Laminar Electrophysiology Probe Into a Predetermined Barrel in Mouse Area S1BF.” Frontiers in Neural Circuits 15:541676. https://doi.org/10.3389/fncir.2021.541676.
Nsiangani, Armel, Joseph Del Rosario, Alan C. Yeh, Donghoon Shin, Shea Wells, Tidhar Lev-Ari, Brice Williams, and Bilal Haider. 2022. “Optimizing Intact Skull Intrinsic Signal Imaging for Subsequent Targeted Electrophysiology across Mouse Visual Cortex.” Scientific Reports 12 (1): 2063. https://doi.org/10.1038/s41598-022-05932-2.
Souza, Bruno Oliveira Ferreira, Mira Abou Rjeili, Clémentine Quintana, Jean M. Beaulieu, and Christian Casanova. 2018. “Spatial Frequency Selectivity Is Impaired in Dopamine D2 Receptor Knockout Mice.” Frontiers in Integrative Neuroscience 11. https://doi.org/10.3389/fnint.2017.00041.
Yeritsyan, Naira, Konrad Lehmann, Oliver Puk, Jochen Graw, and Siegrid Löwel. 2012. “Visual Capabilities and Cortical Maps in BALB/c Mice.” European Journal of Neuroscience 36 (6): 2801–11. https://doi.org/10.1111/j.1460-9568.2012.08195.x.
Zhuang, Jun, Lydia Ng, Derric Williams, Matthew Valley, Yang Li, Marina Garrett, and Jack Waters. 2017. “An Extended Retinotopic Map of Mouse Cortex.” Edited by David Kleinfeld. eLife 6 (January):e18372. https://doi.org/10.7554/eLife.18372.
Don’t miss out! Subscribe to our newsletter for the latest blog updates.
We hope you found this post helpful! If you have any questions or need further information, feel free to reach out. We’re here to help!


